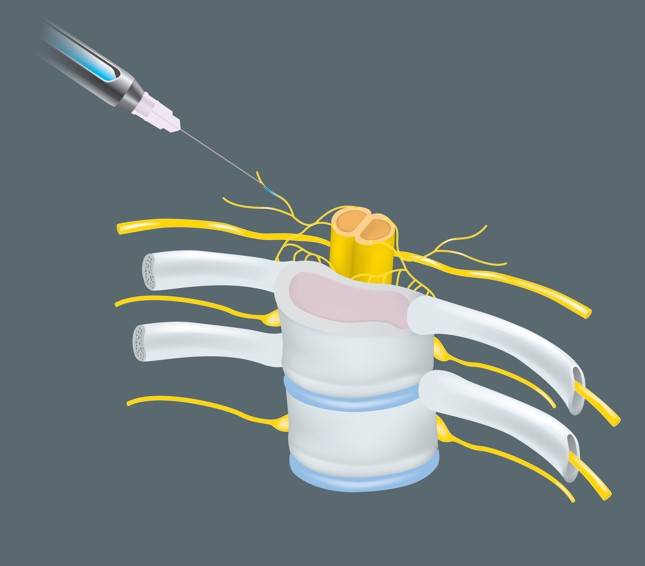
Long-term neurological conditions following nerve blocks due to injury during the procedure are an important topic for anesthesiologists and pain specialists. While extensive research has examined the incidence rates of neurological complications associated with individual risk factors such as type of nerve block, pre-existing neuropathies, intraneural injections, mechanical trauma, anesthetic neurotoxicity, and iatrogenic injuries from surgery, little is known about how the interactions between these factors may change the effects of these conditions. With an incidence rate of 0.24%, these events are extremely rare; however, the morbidity associated with these injuries demands careful consideration and analysis of the contributing factors. The anatomy of peripheral nerves includes several layers of connective tissues, which cover neural axons and provide support and nutrition to the nerves. The outermost layer, the epineurium, encases fascicular bundles within a cushioning tissue known as the interfascicular epineurium. Deeper within the nerve, the perineum coats the axon with a protective layer of cells called the endoneurium. However, this anatomy varies significantly between different nerve types and even along a given nerve.2 Variation in fascicular topography places certain nerve blocks and certain surgeries at higher risk of misplaced block and subsequent neurological conditions.
Although long-term neuropathies occur in 0.2-0.4% of surgeries with nerve blocks, transient neurological symptoms are more likely, occurring in 8.2-15% of surgeries.1 A 2007 meta-analysis found increased likelihood of neuropathy following nerve blocks in areas such as axillary brachial plexus, interscalene, femoral, and sciatic nerves.3 The occurrence of procedure-induced paresthesia is also thought to increase the likelihood of transient neuropathy following surgeries with nerve blocks. Retrospective studies indicate certain types of surgery may also increase the chances of postoperative nerve injury, especially those including trauma, excessive neural stretch, inflammation, or ischemia.1,4
Nerve injury can result from mechanical trauma (e.g. needle injections or pressure injury) or chemical insults (e.g. local anesthetic injection and adjuvant neurotoxicity). Evidence from animal and cadaveric studies indicate long-bevel (14° angle) needles penetrate fascicular bundles through the perineum and have a greater chance of causing nerve injury, compared to short-bevel (45° angle) needles. These findings have increased physician preference of short-bevel needles for surgeries involving nerve blocks. Although all local anesthetics have neurotoxic potential, some are more neurotoxic than others. The neurotoxicity of a local anesthetic is related to prolonged
increases in cytosolic calcium, which leads to mitochondrial injury, a depletion of adenosine triphosphate (ATP), and possibly apoptosis.5
Guidance techniques for nerve block administration can help minimize the risk of postoperative neurological conditions. Modern medical technology allows the use of ultrasound (US) for improved guidance compared to the landmark approach or nerve stimulation (NS). NS techniques first use electrical currents to locate the nerve of interest and later inject anesthetics to induce the block. Several animal studies have noted the inaccuracies of NS in predicting needle tip location at both high- and low-current stimulation.1 In particular, a porcine model showed low-current stimulations could not accurately discern between needle-nerve contact and intraneural injection, increasing the risk of nerve damage and postoperative neural complications.6 US-based techniques can be useful for identifying and avoiding intraneural injection. Some ultrasonographic indicators of intraneural injections include visualization of the needle tip within the nerve, increase in the nerve cross-sectional area by at least 15%, and real-time visualization of fascicle separation on injection. A retrospective review found there was no difference in the incidence of neurological complications for patients receiving nerve blocks under NS or US guidance. However, the incidence of nerve conditions lasting 6-12 months following nerve blocks performed under NS guidance was significantly higher than US-based method.7
The epidemiological triangle is an important framework for understanding the intricate relationships between the agent, host, and environment in the context of injury development. The emergence of neurological conditions following nerve blocks arises from a dynamic interplay of multiple risk factors, with some being essential for causation and others playing a more contributory role. As this field of research moves forward, it is crucial to investigate the nature of these interactions to clarify their role in patient outcomes.
References
1. Sondekoppam, Rakesh V., and Ban C. H. Tsui. “Factors Associated With Risk of Neurologic Complications After Peripheral Nerve Blocks: A Systematic Review.” Anesthesia & Analgesia, vol. 124, no. 2, Feb. 2017, pp. 645–60. https://doi.org/10.1213/ANE.0000000000001804
2. Sunderland, Sydney. “THE INTRANEURAL TOPOGRAPHY OF THE RADIAL, MEDIAN AND ULNAR NERVES.” Brain, vol. 68, no. 4, 1945, pp. 243–98. https://doi.org/10.1093/brain/68.4.243
3. Brull, Richard, et al. “Neurological Complications After Regional Anesthesia: Contemporary Estimates of Risk.” Anesthesia & Analgesia, vol. 104, no. 4, Apr. 2007, pp. 965–74. https://doi.org/10.1213/01.ane.0000258740.17193.ec
4. Neal, Joseph M., et al. “Brachial Plexus Anesthesia: Essentials of Our Current Understanding.” Regional Anesthesia and Pain Medicine 27(4), 2002. 402-428.
5. Kitagawa, Norihito, et al. “Possible Mechanism of Irreversible Nerve Injury Caused by Local Anesthetics.” Anesthesiology, vol. 100, no. 4, Apr. 2004, pp. 962–67, https://doi.org/10.1097/00000542-200404000-00029
6. Wiesmann, Thomas, et al. “Minimal Current Intensity to Elicit an Evoked Motor Response Cannot Discern Between Needle-Nerve Contact and Intraneural Needle Insertion.” Anesthesia & Analgesia, vol. 118, no. 3, Mar. 2014, pp. 681–86. https://doi.org/10.1213/ANE.0b013e3182a94454
7. Orebaugh, Steven L., et al. “Adverse Outcomes Associated With Nerve Stimulator–Guided and Ultrasound-Guided Peripheral Nerve Blocks by Supervised Trainees: Update of a Single-Site Database.” Regional Anesthesia and Pain Medicine, vol. 37, no. 6, 2012, pp. 577–82. https://doi.org/10.1097/AAP.0b013e318263d396