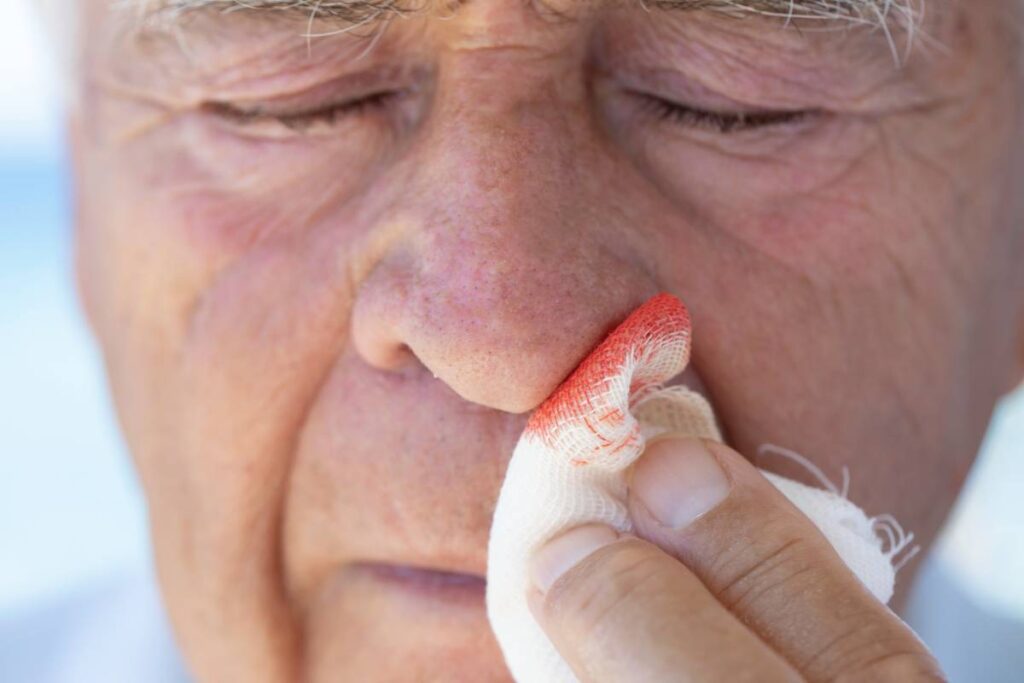
Epistaxis, or nosebleed, occurring during the perioperative period poses significant challenges in clinical management, particularly due to its potential to complicate surgical procedures and recovery. It is a common condition that can result from a variety of causes, including the trauma of nasal surgery, the presence of nasal packing, and the effects of anticoagulant and antiplatelet medications. Effective management is essential to avoid complications such as airway obstruction, increased blood loss, or the need for additional surgical interventions, all of which can prolong hospital stays and increase healthcare costs.
Initial management of perioperative epistaxis typically involves conservative approaches aimed at minimizing trauma and controlling bleeding as early as possible. Common first-line measures include nasal compression, application of ice packs, and use of topical vasoconstrictors such as oxymetazoline or phenylephrine. These interventions can effectively reduce blood flow to the nasal mucosa and are particularly useful in anterior epistaxis, which accounts for the majority of cases (1). Simple anterior nasal packing with gauze or commercially available nasal tampons can also be used. However, packing should be performed carefully to avoid further trauma to the nasal mucosa and to minimize patient discomfort.
For patients in whom conservative measures fail and their nosebleed continues, medical management may be necessary, including the use of antifibrinolytic agents such as tranexamic acid. Tranexamic acid works by inhibiting fibrinolysis, thereby promoting clot stability and reducing bleeding. This approach has been shown to be effective in both anterior and posterior epistaxis and can be administered topically or systemically depending on the clinical scenario (2). In patients receiving anticoagulant or antiplatelet therapy, temporary modification of these medications may be considered. This decision requires careful evaluation of the risk of thromboembolic events versus the benefit of reduced bleeding risk. Typically, discontinuation of antiplatelet therapy or a switch to short-acting agents may be recommended in high-risk cases, with the decision often based on the type and urgency of the procedure, as well as the patient’s overall cardiovascular risk profile.
If bleeding persists despite conservative and medical therapy, surgical intervention may be required. Cauterization is a commonly used technique in the surgical management of epistaxis. Chemical cauterization with silver nitrate is effective for small, visible bleeding points, while electrical cauterization may be used for more extensive bleeding (3). For posterior epistaxis, which tends to be more severe and less accessible through anterior approaches, endoscopic surgical techniques offer a more targeted and effective solution. For example, endoscopic ligation of the sphenopalatine artery has been shown to successfully control bleeding in refractory cases and is associated with a low recurrence rate.
In severe cases of refractory epistaxis, especially those that do not respond to other surgical interventions, arterial embolization may be considered. This minimally invasive procedure involves the occlusion of the offending vessel under imaging guidance, thereby stopping the blood flow to the site of bleeding. Although effective, embolization carries risks such as stroke or tissue necrosis and is typically reserved for cases where other treatments have failed (4).
Overall, the management of perioperative epistaxis requires a thorough understanding of the patient’s individual risk factors and the use of a stepwise approach that progresses from conservative management to more invasive interventions as needed. By implementing appropriate preventive measures and treating bleeding episodes promptly, healthcare providers can reduce the impact of epistaxis on surgical outcomes and patient safety.
References
- Beck R, Sorge M, Schneider A, Dietz A. Current Approaches to Epistaxis Treatment in Primary and Secondary Care. Dtsch Arztebl Int. 2018;115(1-02):12-22. doi:10.3238/arztebl.2018.0012
- Musgrave KM, Powell J. A systematic review of anti-thrombotic therapy in epistaxis. Rhinology. 2016;54(4):292-391. doi:10.4193/Rhino16.040
- Escabasse V, Bequignon E, Vérillaud B, et al. Guidelines of the French Society of Otorhinolaryngology (SFORL). Managing epistaxis under coagulation disorder due to antithrombotic therapy. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134(3):195-199. doi:10.1016/j.anorl.2016.10.001
- Liu X, Wang P, Li M, Chen G. Incidence, risk factors, management and prevention of severe postoperative epistaxis after endoscopic endonasal transsphenoidal surgery: a single center experience. Front Surg. 2023;10:1203409. Published 2023 Jul 26. doi:10.3389/fsurg.2023.1203409